Abstract
Introduction: There is a significant unmet need for the development of reliable genetic biomarkers that identify patients with newly-diagnosed primary CNS lymphoma (PCNSL) who are at high risk of progression, both during standard methotrexate-based induction as well as delayed progression after receipt of dose-intensive consolidation. Currently, little is known about the genetic subtypes of PCNSL and the basis for differences in chemo-immunotherapeutic response. We hypothesized that an integrated clinical-genomic analysis may reveal new determinants of tumor progression and may ultimately guide a precision medicine-based approach to the selection of effective treatment regimens.
Methods: We performed targeted next-generation sequencing of 529 pan-cancer genes of pre-treatment, formalin-fixed, paraffin-embedded tumors from 52 patients with CNS diffuse large B-cell lymphoma. We identified structural variants including single nucleotide variants, small insertions and deletions, and copy-number alterations. Clinical records were retrospectively reviewed to determine patients' baseline characteristics, IELSG index, and progression-free survival (PFS). 44 EBV-negative PCNSL patients treated with methotrexate, temozolomide, rituximab (MTR) induction with adequate genomic samples were available for complete analysis. Statistical testing was performed using R.
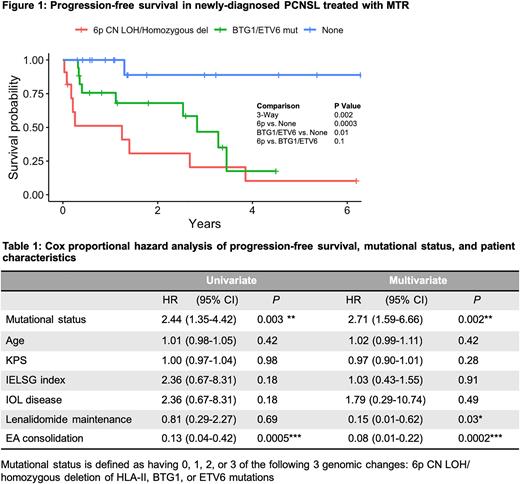
Results: We identified three candidate genomic alterations that were significantly associated with chemotherapy resistance in PCNSL: (1) Alterations at 6p21.3, including both copy neutral loss of heterozygosity (CN LOH) at the HLA Class I (HLA-A, B, C) and Class II loci (HLA-DR, DP, DQ, TAP1) as well as homozygous deletion of HLA Class II; (2) Mutations of tumor suppressor genes BTG-1 and ETV6 (Fisher's exact, p < 0.01). Overall, these genomic alterations were detected in 18 out of 19 progression events in the cohort (Fisher's exact, p < 0.001). 7 of 9 tumors with 6p CN LOH also contained mutations at BTG-1 and/or ETV6. Kaplan-Meier survival analysis shows three subgroups stratified by alteration type with distinct PFS outcomes (Figure 1). The subgroup with either CN LOH or homozygous deletion at 6p had the earliest progression, followed by the subgroup with mutations at either BTG-1/ETV6 and no 6p CN LOH or homozygous deletion, compared to no alterations at these 3 loci (Log-rank, p < 0.01). Patients with hemizygous loss at 6p exhibited a trend towards worse outcome compared to no 6p loss. Furthermore, multivariate Cox proportional hazard analysis demonstrated a statistically significant 2.7-fold increased risk of progression with 6p CN LOH/homozygous deletion or mutations involving BTG-1/ETV6 (Table 1). While etoposide-cytarabine consolidation and maintenance lenalidomide were each significantly associated with a decreased risk of progression, age at diagnosis, Karnofsky performance status, intraocular disease status, and IELSG index were not significantly associated with PFS.
Conclusions: Through harmonizing clinical and next-generation sequencing data, in this retrospective analysis we identify pathogenic alterations involving BTG-1 and ETV6, as well as major alterations at 6p21.3, that correlated with accelerated disease progression and an early tumor resistance phenotype in PCNSL. While 6p loss has been identified as a mechanism of tumor immune escape in PCNSL, our results suggest that 6p CN LOH may be a novel mechanism associated with early progression. Of note, in a parallel investigation using patient-derived CNS lymphoma tumor cells engrafted in a murine orthotopic xenograft model, we have demonstrated that lenalidomide significantly induces transcription of 6p21.3 genes involved in antigen presentation and immunomodulation, including HLA-A, TAP-1, and lymphotoxins. Taken together, 6p21.3 copy number loss, in particular CN LOH and homozygous deletion, as well as mutations in BTG-1 and ETV6 may be a promising biomarker set, potentially useful in risk stratification in clinical trials as well as to identify high risk patients more likely to derive benefit from lenalidomide. Prospective validation studies are planned to confirm these results.
Supported by NCI.
Disclosures
Rubenstein:NURIX: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.